Page 30 - IJB-7-3
P. 30
3D Bioprinted Organoids
A B
E C
D
F
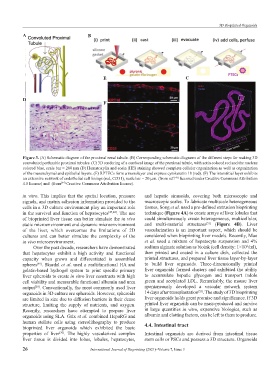
Figure 3. (A) Schematic diagram of the proximal renal tubule. (B) Corresponding schematic diagrams of the different steps for making 3D
convoluted perfusable proximal tubules. (C) 3D rendering of a confocal image of the proximal tubule, with actin colored red and the nucleus
colored blue, scale bar = 200 µm (D) Hematoxylin and eosin (HE) staining showed complete cellular organization as well as organization
of the mesenchymal and epithelial layers. (E) RPTECs form a monolayer and express cytokeratin 18 (red). (F) The interstitial layer exhibits
an extensive network of endothelial cell linings (red, CD31), scale bar = 20 µm. (from ref. licensed under Creative Commons Attribution
[43]
4.0 license) and (from Creative Commons Attribution license).
[44]
in vitro. This implies that the spatial location, pressure and hepatic sinusoids, covering both microscopic and
signals, and matrix adhesion information provided to the macroscopic scales. To fabricate multiscale heterogeneous
cells in a 3D culture environment play an important role tissues, Song et al. used a pre-defined extrusion bioprinting
in the survival and function of hepatocytes [47,48] . The use technique (Figure 4A) to create arrays of liver lobules that
of bioprinted liver tissue can better simulate the in vivo could simultaneously create heterogeneous, multicellular,
[51]
static microenvironment and dynamic microenvironment and multi-material structures (Figure 4B). Liver
of the liver, which overcomes the limitations of 2D vascularization is an important aspect, which should be
cultures and can better simulate the complexity of the considered when bioprinting liver models. Recently, Mao
in vivo microenvironment. et al. used a mixture of hepatocyte suspension and 4%
6
Over the past decade, researchers have demonstrated sodium alginate solution as bioink (cell density: 1×10 /ml),
that hepatocytes exhibit a high activity and functional then printed and coated in a culture dish, collected the
capacity when grown and differentiated in assembled printed structures, and prepared liver tissue layer-by-layer
spheres . Skardal et al. used a multifunctional HA and to build liver organoids. Three-dimensionally printed
[49]
gelatin-based hydrogel system to print specific primary liver organoids formed clusters and exhibited the ability
liver spheroids to create in vitro liver constructs with high to accumulate hepatic glycogen and transport indole
cell viability and measurable functional albumin and urea green and acetylated LDL. Remarkably, the mouse liver
output . Conventionally, the most commonly used liver spontaneously developed a vascular network system
[28]
[52]
organoids in 3D culture are spheroids. However, spheroids 14 days after transplantation . The study of 3D bioprinting
are limited in size due to diffusion barriers in their dense liver organoids holds great promise and significance. If 3D
structure, limiting the supply of nutrients, and oxygen. printed liver organoids can be mass-produced and survive
Recently, researchers have attempted to prepare liver in large quantities in vitro, expensive biologics, such as
organoids using SLA. Grix et al. combined HepaRG and albumin and clotting factors, can be left to them to produce.
human stellate cells using stereolithography to produce 4.4. Intestinal tract
bioprinted liver organoids which exhibited the basic
properties of liver . The highly vascularized complex Intestinal organoids are derived from intestinal tissue
[50]
liver tissue is divided into lobes, lobules, hepatocytes, stem cells or PSCs and possess a 3D structure. Organoids
26 International Journal of Bioprinting (2021)–Volume 7, Issue 3