Page 390 - IJB-9-5
P. 390
International Journal of Bioprinting Vascularized bone regeneration
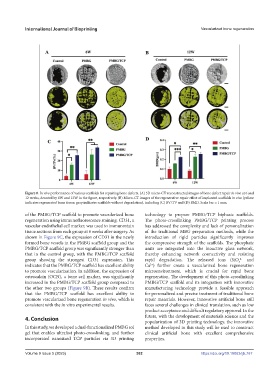
Figure 8. In vivo performance of various scaffolds for repairing bone defects. (A) 3D micro-CT reconstructed images of bone defect repair in vivo at 6 and
12 weeks, denoted by 6W and 12W in the figure, respectively. (B) Micro-CT images of the regenerative repair effect of implanted scaffolds in vivo (yellow
indicates regenerated bone tissue; gray indicates scaffolds without degradation), including (C) BV/TV and (D) BMD. Scale bar = 1 mm.
of the PMBG/TCP scaffold to promote vascularized bone technology to prepare PMBG/TCP biphasic scaffolds.
regeneration using immunofluorescence staining. CD31, a The photo-crosslinking PMBG/TCP printing process
vascular endothelial cell marker, was used to immunostain has addressed the complexity and lack of personalization
tissue sections from each group at 6 weeks after surgery. As of the traditional MBG preparation methods, while the
shown in Figure 9C, the expression of CD31 in the newly introduction of rigid particles significantly improves
formed bone vessels in the PMBG scaffold group and the the compressive strength of the scaffolds. The phosphate
PMBG/TCP scaffold group was significantly stronger than units are integrated into the bioactive glass network,
that in the control group, with the PMBG/TCP scaffold thereby enhancing network connectivity and resisting
group showing the strongest CD31 expression. This rapid degradation. The released ions (SiO and
4-
4
indicates that the PMBG/TCP scaffold has excellent ability Ca ) further create a vascularized bone regeneration
2+
to promote vascularization. In addition, the expression of microenvironment, which is crucial for rapid bone
osteocalcin (OCN), a bone cell marker, was significantly regeneration. The development of this photo-crosslinking
increased in the PMBG/TCP scaffold group compared to PMBG/TCP scaffold and its integration with innovative
the other two groups (Figure 9B). These results confirm manufacturing technology provide a feasible approach
that the PMBG/TCP scaffold has excellent ability to for personalized and precise treatment of traditional bone
promote vascularized bone regeneration in vivo, which is repair materials. However, innovative artificial bone still
consistent with the in vitro experimental results. faces several challenges in clinical translation, such as low
product acceptance and difficult regulatory approval. In the
4. Conclusion future, with the development of materials science and the
popularization of 3D printing technology, the innovative
In this study, we developed a dual-functionalized PMBG sol method developed in this study will be used to construct
gel that enables ultrafast photo-crosslinking, and further clinical artificial bone with excellent comprehensive
incorporated nanosized TCP particles via 3D printing properties.
Volume 9 Issue 5 (2023) 382 https://doi.org/10.18063/ijb.767