Page 555 - IJB-9-6
P. 555
International Journal of Bioprinting 3D printing of PCL-ceramic composite scaffolds
the composite scaffolds. The decreasing order of contact 3.6. Cell viability on 3D-printed scaffolds
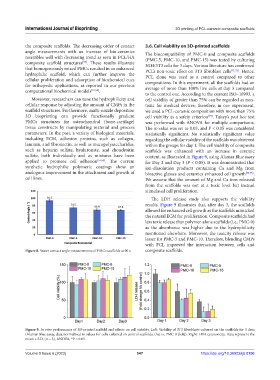
angle measurements with an increase of bio-ceramics The biocompatibility of PMC-0 and composite scaffolds
resembles well with decreasing trend as seen in PCL/HA (PMC-5, PMC-10, and PMC-15) was tested by culturing
composite scaffold structures . These results illustrate NIH/3T3 cells for 3 days. Various literature has confirmed
[59]
that homogeneously mixed PMCs resulted in an enhanced PCL’s non-toxic effect on 3T3 fibroblast cells [71,72] . Hence,
hydrophilic scaffold, which can further improve the PCL alone was used as a control compared to other
cellular proliferation and adsorption of biochemical cues compositions. In this experiment, all the scaffolds had an
for orthopedic applications, as reported in our previous average of more than 100% live cells at day 3 compared
computational biochemical models [31,55] .
to the control one. According to the current ISO–10993, a
Moreover, researchers can tune the hydrophilicity and cell viability of greater than 75% can be regarded as non-
cellular response by adjusting the amount of CMPs in the toxic for medical devices; therefore, in our experiment,
scaffold structures. Furthermore, multi-nozzle deposition we used a PCL-ceramic composition with more than 75%
3D bioprinting can provide functionally gradient cell viability as a safety criterion . Tukey’s post hoc test
[73]
PMCs structures for osteochondral (bone-cartilage) was performed with ANOVA for multiple comparisons.
tissue constructs by manipulating material and process The α-value was set to 0.05, and P < 0.05 was considered
parameters. In the past, a variety of biological materials, statistically significant. No statistically significant value
including ECM, adhesion proteins, such as collagen, regarding the cellular viability of the scaffolds was observed
laminin, and fibronectin, as well as mucopolysaccharides, within the groups for day 1. The cell viability of composite
such as heparin sulfate, hyaluronate, and chondroitin scaffolds was enhanced with an increase in ceramic
sulfate, both individually and as mixtures have been content, as illustrated in Figure 9, using Alamar Blue assay
applied to promote cell adhesion [51,53] . The current for Day 2 and Day 3 (P < 0.05). It was demonstrated that
synthetic hydrophilic polymeric coatings show an ion-dissolution products containing Ca and Mg from
analogous improvement in the attachment and growth of bioactive glasses and ceramics enhanced cell growth [74,75] .
cell lines. We assume that the amount of Mg and Ca ions released
from the scaffolds was not at a toxic level but instead
stimulated cell proliferation.
The LDH release study also supports the viability
results. Figure 9 illustrates that, after day 3, the scaffolds
allowed for enhanced cell growth as the scaffolds mimicked
the natural ECM for proliferation. Composite scaffolds had
less toxic release than polymer-alone scaffolds (i.e., PMC-0)
as the absorbance was higher due to the hydrophilicity
mentioned elsewhere. Moreover, the toxicity release was
lesser for PMC-5 and PMC-10. Therefore, blending CMPs
with PCL improved the interaction between cells and
Figure 8. Water contact angle measurements of PMCs scaffolds at 90 s. composite scaffolds.
Figure 9. In vitro performance of 3D-printed scaffold and effects on cell viability. Left: Viability of 3T3 fibroblasts cultured on the scaffolds for 3 days
(Alamar Blue assay, data normalized to values for cells cultured on control scaffolds, that is, PMC-0 [left]). Right: LDH cytotoxicity. Data represent the
mean ± S.D. (n = 3), ANOVA, *P < 0.05.
Volume 9 Issue 6 (2023) 547 https://doi.org/10.36922/ijb.0196