Page 80 - TD-3-1
P. 80
Tumor Discovery Missense mutations in CXCR1: Impact on stability and function
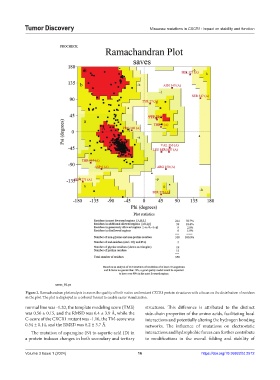
Figure 2. Ramachandran plot analysis to assess the quality of both native and mutant CXCR1 protein structures with a focus on the distribution of residues
in the plot. The plot is displayed in a colored format to enable easier visualization.
normal line was -1.20, the template modeling score (TMS) structures. This difference is attributed to the distinct
was 0.56 ± 0.15, and the RMSD was 6.4 ± 3.9 Å, while the side-chain properties of the amino acids, facilitating local
C-score of the CXCR1 mutant was -1.10, the TM-score was interactions and potentially altering the hydrogen bonding
0.54 ± 0.14, and the RMSD was 6.2 ± 3.7 Å. networks. The influence of mutations on electrostatic
The mutation of asparagine (N) to aspartic acid (D) in interactions and hydrophobic forces can further contribute
a protein induces changes in both secondary and tertiary to modifications in the overall folding and stability of
Volume 3 Issue 1 (2024) 16 https://doi.org/10.36922/td.2512